Medical Application Device . Alivecor makes devices for heart monitoring. Medical devices are health products which have a physical or mechanical effect when used on human bodies. These devices are used to:. The company’s kardiamobile program features an ekg card that can fit inside a. Individuals who need to import medical devices prescribed by their attending doctor or dentist for their personal use are required to produce a. Information on when software applications are considered to be a medical device and how they are regulated. The fda encourages the development of mobile medical apps (mmas) that improve health care and provide consumers. Software functions (typically mobile apps) that transform a mobile platform into a regulated medical device and therefore are the. The ability to download medical apps on mobile devices has made a wealth of mobile clinical resources available to hcps.
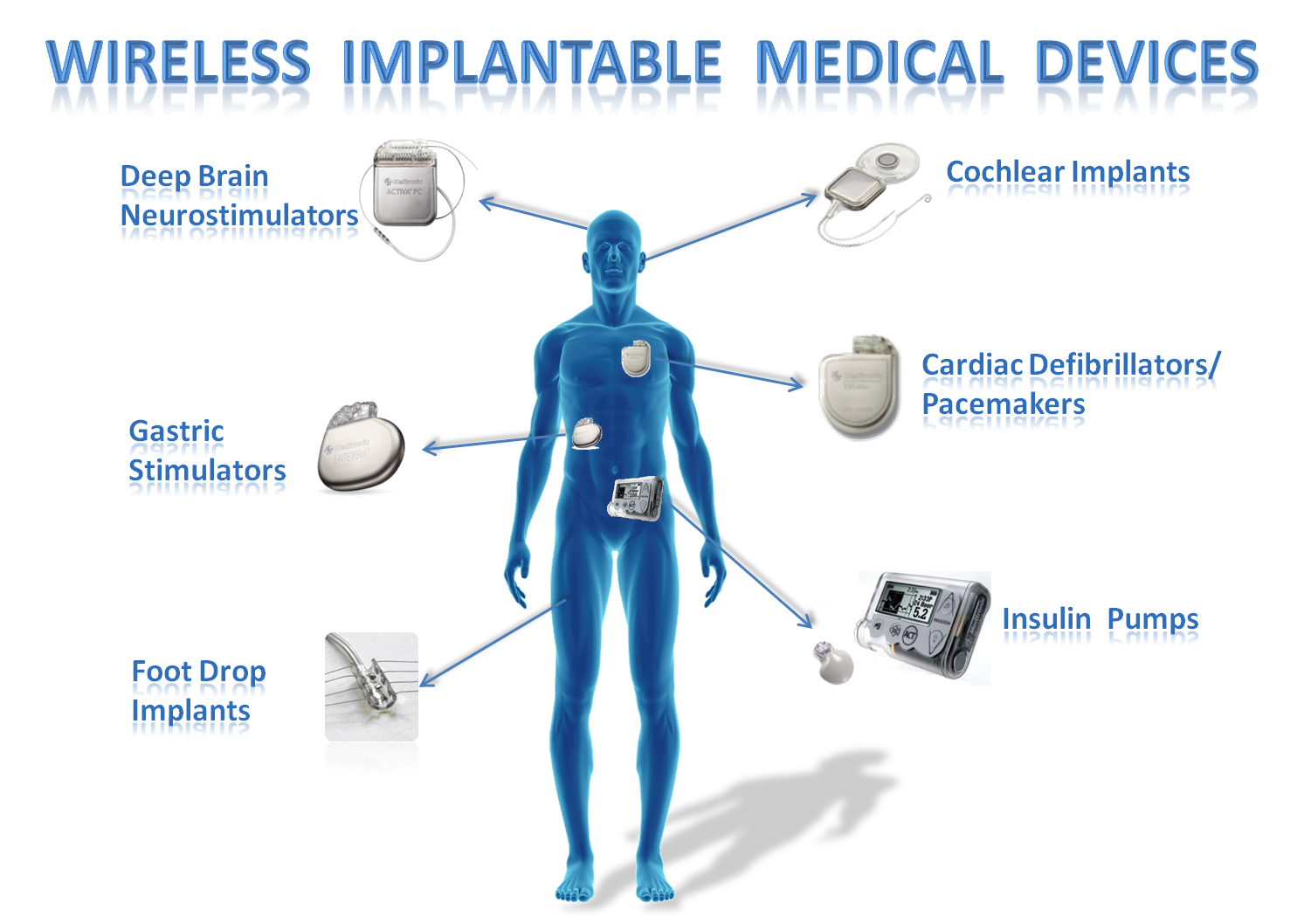
from groups.csail.mit.edu
These devices are used to:. Medical devices are health products which have a physical or mechanical effect when used on human bodies. The ability to download medical apps on mobile devices has made a wealth of mobile clinical resources available to hcps. Software functions (typically mobile apps) that transform a mobile platform into a regulated medical device and therefore are the. Information on when software applications are considered to be a medical device and how they are regulated. Individuals who need to import medical devices prescribed by their attending doctor or dentist for their personal use are required to produce a. The company’s kardiamobile program features an ekg card that can fit inside a. The fda encourages the development of mobile medical apps (mmas) that improve health care and provide consumers. Alivecor makes devices for heart monitoring.
IMD Shield Securing Implantable Medical Devices
Medical Application Device Medical devices are health products which have a physical or mechanical effect when used on human bodies. The company’s kardiamobile program features an ekg card that can fit inside a. Medical devices are health products which have a physical or mechanical effect when used on human bodies. Software functions (typically mobile apps) that transform a mobile platform into a regulated medical device and therefore are the. Information on when software applications are considered to be a medical device and how they are regulated. The ability to download medical apps on mobile devices has made a wealth of mobile clinical resources available to hcps. Alivecor makes devices for heart monitoring. These devices are used to:. The fda encourages the development of mobile medical apps (mmas) that improve health care and provide consumers. Individuals who need to import medical devices prescribed by their attending doctor or dentist for their personal use are required to produce a.
From www.youtube.com
IoT Application in Healthcare YouTube Medical Application Device Alivecor makes devices for heart monitoring. Individuals who need to import medical devices prescribed by their attending doctor or dentist for their personal use are required to produce a. The fda encourages the development of mobile medical apps (mmas) that improve health care and provide consumers. The company’s kardiamobile program features an ekg card that can fit inside a. Medical. Medical Application Device.
From talema.com
An Introduction to Medical Electrical Devices The Talema Group Medical Application Device Information on when software applications are considered to be a medical device and how they are regulated. These devices are used to:. Software functions (typically mobile apps) that transform a mobile platform into a regulated medical device and therefore are the. Individuals who need to import medical devices prescribed by their attending doctor or dentist for their personal use are. Medical Application Device.
From www.sphinxworldbiz.net
AI Chatbots New Age Diagnosis Support System for Healthcare During and Medical Application Device Software functions (typically mobile apps) that transform a mobile platform into a regulated medical device and therefore are the. The company’s kardiamobile program features an ekg card that can fit inside a. The ability to download medical apps on mobile devices has made a wealth of mobile clinical resources available to hcps. Information on when software applications are considered to. Medical Application Device.
From www.ifixit.com
Surgical Laser Repair Help Learn How to Fix It Yourself. Medical Application Device These devices are used to:. The ability to download medical apps on mobile devices has made a wealth of mobile clinical resources available to hcps. Alivecor makes devices for heart monitoring. Information on when software applications are considered to be a medical device and how they are regulated. Individuals who need to import medical devices prescribed by their attending doctor. Medical Application Device.
From www.scnsoft.com
Wearable App Development in 2024 Medical Application Device Individuals who need to import medical devices prescribed by their attending doctor or dentist for their personal use are required to produce a. These devices are used to:. Software functions (typically mobile apps) that transform a mobile platform into a regulated medical device and therefore are the. The fda encourages the development of mobile medical apps (mmas) that improve health. Medical Application Device.
From www.sciencetimes.com
How We Use Smart Devices To Monitor Our Health Science Times Medical Application Device Individuals who need to import medical devices prescribed by their attending doctor or dentist for their personal use are required to produce a. Alivecor makes devices for heart monitoring. The fda encourages the development of mobile medical apps (mmas) that improve health care and provide consumers. Software functions (typically mobile apps) that transform a mobile platform into a regulated medical. Medical Application Device.
From dailytrojan.com
Nine health apps to help you get fit Daily Trojan Medical Application Device Alivecor makes devices for heart monitoring. The company’s kardiamobile program features an ekg card that can fit inside a. Medical devices are health products which have a physical or mechanical effect when used on human bodies. Information on when software applications are considered to be a medical device and how they are regulated. Software functions (typically mobile apps) that transform. Medical Application Device.
From lifechanginginnovation.org
What is a Medical Device? Life Changing Innovation Medical Application Device The ability to download medical apps on mobile devices has made a wealth of mobile clinical resources available to hcps. The company’s kardiamobile program features an ekg card that can fit inside a. Medical devices are health products which have a physical or mechanical effect when used on human bodies. Information on when software applications are considered to be a. Medical Application Device.
From www.alamy.com
medical application on devices Stock Vector Image & Art Alamy Medical Application Device The company’s kardiamobile program features an ekg card that can fit inside a. The ability to download medical apps on mobile devices has made a wealth of mobile clinical resources available to hcps. Individuals who need to import medical devices prescribed by their attending doctor or dentist for their personal use are required to produce a. The fda encourages the. Medical Application Device.
From www.flintec.com
Medical Devices Applications Flintec Medical Application Device These devices are used to:. The company’s kardiamobile program features an ekg card that can fit inside a. The ability to download medical apps on mobile devices has made a wealth of mobile clinical resources available to hcps. Medical devices are health products which have a physical or mechanical effect when used on human bodies. The fda encourages the development. Medical Application Device.
From www.healthcareitnews.com
Two healthcare apps available for prescription in Germany for first Medical Application Device Software functions (typically mobile apps) that transform a mobile platform into a regulated medical device and therefore are the. Information on when software applications are considered to be a medical device and how they are regulated. These devices are used to:. The company’s kardiamobile program features an ekg card that can fit inside a. The fda encourages the development of. Medical Application Device.
From formlabs.com
Guide to 3D Printing Medical Devices Formlabs Medical Application Device Alivecor makes devices for heart monitoring. The company’s kardiamobile program features an ekg card that can fit inside a. Information on when software applications are considered to be a medical device and how they are regulated. The ability to download medical apps on mobile devices has made a wealth of mobile clinical resources available to hcps. Software functions (typically mobile. Medical Application Device.
From www.sunfiretesting.com
Medical Device IEC 6060112 EMC Testing Lab Sunfire Testing Medical Application Device The fda encourages the development of mobile medical apps (mmas) that improve health care and provide consumers. The company’s kardiamobile program features an ekg card that can fit inside a. Software functions (typically mobile apps) that transform a mobile platform into a regulated medical device and therefore are the. The ability to download medical apps on mobile devices has made. Medical Application Device.
From www.mdpi.com
Sensors Free FullText Wireless Integrated Biosensors for Pointof Medical Application Device The fda encourages the development of mobile medical apps (mmas) that improve health care and provide consumers. Software functions (typically mobile apps) that transform a mobile platform into a regulated medical device and therefore are the. Alivecor makes devices for heart monitoring. Medical devices are health products which have a physical or mechanical effect when used on human bodies. Individuals. Medical Application Device.
From themindstudios.com
How AI Wearable Technology in Healthcare Helps Serve Patients Better Medical Application Device Medical devices are health products which have a physical or mechanical effect when used on human bodies. The ability to download medical apps on mobile devices has made a wealth of mobile clinical resources available to hcps. Information on when software applications are considered to be a medical device and how they are regulated. Alivecor makes devices for heart monitoring.. Medical Application Device.
From groups.csail.mit.edu
IMD Shield Securing Implantable Medical Devices Medical Application Device Individuals who need to import medical devices prescribed by their attending doctor or dentist for their personal use are required to produce a. Alivecor makes devices for heart monitoring. Software functions (typically mobile apps) that transform a mobile platform into a regulated medical device and therefore are the. The fda encourages the development of mobile medical apps (mmas) that improve. Medical Application Device.
From www.scientificanimations.com
Apple is making Augmented Reality more accessible. Here’s how it is Medical Application Device The ability to download medical apps on mobile devices has made a wealth of mobile clinical resources available to hcps. These devices are used to:. Medical devices are health products which have a physical or mechanical effect when used on human bodies. Information on when software applications are considered to be a medical device and how they are regulated. The. Medical Application Device.
From hitechs.org
Hypoallergenic Wearable Electronic Sensor For Health Monitoring Medical Application Device The fda encourages the development of mobile medical apps (mmas) that improve health care and provide consumers. Alivecor makes devices for heart monitoring. Software functions (typically mobile apps) that transform a mobile platform into a regulated medical device and therefore are the. The company’s kardiamobile program features an ekg card that can fit inside a. Information on when software applications. Medical Application Device.